Tri-Prime Gene's R&D center adheres to the original concept of technology, has developed over thirty years, and has successively established high-efficiency expression and purification technology platforms for recombinant protein drugs, high-efficiency, safe, and new type of interferon preparation technology platforms, as well as universal cancer treatment technology platforms. In 2011, it was recognized by the Beijing Municipal Science and Technology Commission as the Beijing Municipal Long-term Interferon Engineering Technology Research Center. Tri-Prime Gene's development has independent intellectual property rights of a new class of drug human interferon α1b (Hapgen®), obtaining more than ten new drug certificates and production approval documents, and being rated as national key new products, Beijing municipal autonomous innovation products, and Zhongguancun National Independent Innovation Demonstration Zone's new technological new products.
Tri-Prime Gene's R&D center was approved by the Ministry of Personnel in 2001 to establish a post-doctoral research station, serving as a base for cultivating talents in life sciences and technology, accumulating nearly a hundred graduate students and post-doctoral researchers. In 2018, Tri-Prime Gene was approved by the Beijing Science and Technology Association to become an academician workstation. Tri-Prime Gene has undertaken more than twenty national and municipal key technology projects, obtaining more than fifty invention patents from China, the United States, South Korea, Japan, and other countries, forming a sustainable growth model driven by talents.


Through optimization of vectors for the recombinant protein expression systems in Escherichia coli, optimization of fermentation processes, and purification process improvements, we have taken the lead in China to innovatively produce a variety of interferon subtypes including human interferons α1b, α2a, α2b, breaking the market monopoly and high prices of imported interferons in the Chinese market.


By establishing a high-stability water solution technology platform for recombinant proteins, we have successfully developed water injections, nebulizers, drops, pre-filled syringes, and various dosage forms of interferon subtypes including human interferons α1b, α2a, α2b. The research has received support from the National High-Tech Research and Development Plan (863 Program), the "Eleventh Five-Year Plan" Major New Drug Innovation Projects, and has been awarded multiple Chinese invention patents. Among them, "A Stable Recombinant Human Interferon α1b Water Solution" (ZL 200410069390.4) has won the second prize at the 20th China Invention Exhibition. Additionally, by establishing an inhalation formulation technology platform for atomized absorption of interferon and dry powder inhalation, we have successfully developed dry powder inhalers and other formulation products, as well as clinical application solutions for combined drug absorption, securing multiple invention patents.


Currently, the long-acting technology of protein drugs mainly relies on polyethylene glycol (PEG) modification and albumin fusion expression, with industrial applications already realized for imported long-acting antagonists (first-generation long-acting antagonists) and several domestically developed long-acting antagonists that also use PEG modification technology. The drawback is that PEG molecules randomly modify multiple sites on the drug, making it difficult to separate the modified heterogeneous mixture, resulting in low yield and high cost.
The innovative PEG specific point modification technology introduced by Tri-Prime Gene introduces a semi-peptide amino acid residue at the specific site of the drug, allowing maleic anhydride (MAL) activated PEG molecules to specifically bind to the site, achieving specific point modification. This simplifies the post-modification purification process, increases product yield, unifies structure, and is easy to control quality, significantly reducing manufacturing costs. It is a comprehensively superior innovative protein drug with excellent performance. The "Antagonist α mutant body and its PEG derivative" PCT patent technology (PCT/CN2007/003711) has been authorized in the United States, Japan, South Korea, and China. Additionally, 14 formulation research and analysis technologies have obtained Chinese invention patent authorizations (ZL 201010149849.7, ZL 201110339619.1, ZL 201210043375.7, ZL 201210043371.9, ZL 201210043687.8, ZL 201210043686.3, ZL 201210043688.2, ZL 201210043689.7, ZL 201210043385.0).
In 2012, Tri-Prime Gene was recognized by the Beijing Municipal Science Commission as the "Beijing Municipal Long-Acting Antagonist Engineering Technology Research Center." Based on the above new technology development, the "Polyethylene Glycol New Type Long-Acting Antagonist Mutant Body Injection" has entered the clinical research phase.


Tri-Prime Gene will design and establish a cellular therapy engineering center integrated into the company's new factory area for intelligent production and R&D base project construction. This platform possesses natural γδT cell activation and expansion core technologies, capable of increasing γδT cell proliferation by 450 times to 16,000 times, providing important support for multiple clinical research infusions. The platform aims to develop "off-the-shelf" cellular therapy products through technological innovation, significantly reducing costs and enhancing clinical drug availability.
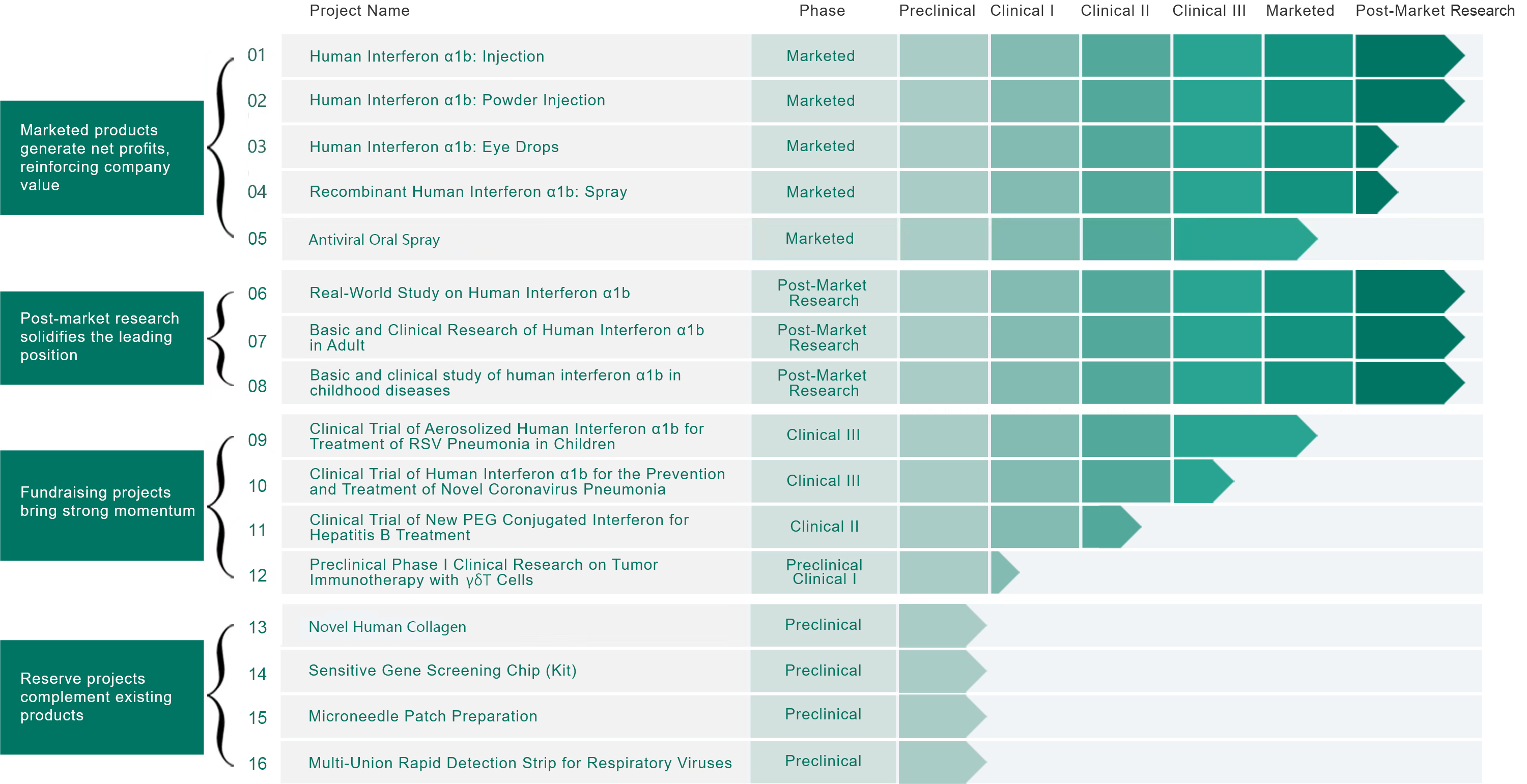
RSV is the primary virus causing respiratory infections in infants and young children, presenting a significant clinical challenge due to its severe impact on patients and the lack of effective therapeutic options. The development of recombinant human interferonα1b as an inhalable treatment will provide patients with safe, effective, and convenient medications for treating RSV pneumonia. Tri-Prime Gene relies on its heavy protein drug high-expression and purification technology platform and heavy protein drug series formulation technology platform, establishing comprehensive production quality standards and control systems, ensuring the effectiveness and safety of the drugs. This platform will provide important support for the prevention and treatment of viral infectious diseases in children's respiratory tracts.
Tri-Prime Gene has established unmet clinical needs for COVID-19, focusing on providing safe and effective treatment options for low-age infected children and vulnerable groups at risk of exposure. Through the development of recombinant human interferonα1b as an inhalable treatment, Tri-Prime Gene leverages its heavy protein drug high-expression and purification technology platform and heavy protein drug series formulation technology platform, establishing comprehensive production quality standards and control systems, ensuring the effectiveness and safety of the drugs. Tri-Prime Gene closely monitors the epidemic characteristics and pathogenic traits of novel coronaviruses, optimizing recombinant human interferonα1b for preventing and treating COVID-19. Clinical trials have shown early intervention can effectively prevent the replication of novel coronaviruses, reducing the incidence and transmission of post-infection complications.
In the field of chronic hepatitis B, addressing the serious issues of low cure rates and ineffective treatments, Tri-Prime Gene provides precise diagnostic and treatment solutions through two main aspects. First, it vigorously promotes the detection of relevant genes for interferon sensitivity to accurately screen suitable populations for interferon therapy. Second, it accelerates the clinical research on new high-efficiency and long-acting interferons that are safe, effective, and efficient, aiming to bring these products to market quickly. This combination plan aligns with national guidelines on hepatitis B treatment in China over the past decade, taking clinical practice as the primary goal for precise treatment. Relying on the "Heavy Protein Drug High-Expression and Purification Technology Platform," "High-Efficiency, Long-Acting, Safe New Type Interferon Preparation Technology Platform," and the "Beijing Municipal Key Laboratory of Interferon Engineering Technology Research Center," Tri-Prime Gene has developed a new gene prediction method for interferon efficacy, significantly improving the accuracy of HBeAg seroconversion prediction in hepatitis B patients, actively promoting the clinical application of novel long-acting interferons and combining them with gene testing to establish an internationally leading model for precise diagnosis and treatment of hepatitis B in China.
Immune cell therapy is a novel and highly effective treatment modality for killing malignant tumor cells. Tri-Prime Gene, in collaboration with the Chinese Academy of Medical Sciences, discovered that γδT cells combined with recombinant human interferonα1b exhibit enhanced anti-tumor effects. Tri-Prime Gene, leveraging its internationally advanced γδT cell therapy technology and partnerships with companies specializing in γδT cell therapy, jointly developed a new method for treating blood tumors and solid tumors with γδT cells. The safety profile of γδT cell therapy is excellent. Tri-Prime Gene relies on its intelligent production base and other generic immune cell therapy technology platforms to focus on the research and development of high-quality generic cell therapy products.